Batteries
Batteries are widely used as sources of direct current electrical energy in automobiles, boats, aircraft, ships, portable electric/electronic equipment, and lighting equipment.
In some instances, batteries are used as the only source of power; while in others, they are used as a secondary or standby power source.
They consists of a number of cells assembled in a common container and connected together to function as a source of electrical power.
The cell is a device that transforms chemical energy into
electrical energy. The simplest cell, known as either a galvanic or voltaic
cell, is shown in the illustration below. It consists of a piece of carbon (C)
and a piece of zinc (Zn) suspended in a jar that contains a solution of water
(H20) and sulfuric acid (H2S0 4) called the electrolyte.
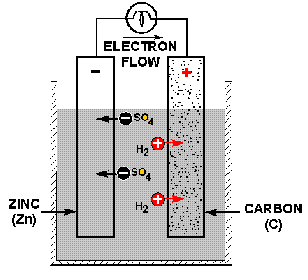
An illustration of a simple galvanic or voltaic cell.
The cell is the fundamental unit. A simple cell consists of two electrodes placed in a container that holds the electrolyte.
In some cells the container acts as one of the electrodes and, in this case, is acted upon by the electrolyte. The electrodes are the conductors by which the current leaves or returns to the electrolyte.
In the simple cell, they are carbon and zinc strips that are
placed in the electrolyte; while in the dry cell (illustrated below), they are
the carbon rod in the center and zinc container in which the cell is assembled.
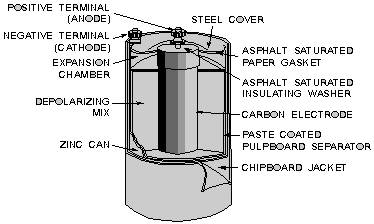
Cutaway view of the general-purpose dry cell.
The electrolyte is the solution that acts upon the electrodes. The electrolyte, which provides a path for electron flow, may be a salt, an acid, or an alkaline solution.
In the simple galvanic cell, the electrolyte is in a liquid form. In the dry cell, the electrolyte is a paste.
The container which may be constructed of one of many different materials provides a means of holding (containing) the electrolyte. The container is also used to mount the electrodes.
In the voltaic cell the container must be constructed of a material that will not be acted upon by the electrolyte.
In a battery there are two cells, (a primary, and a secondary).
A primary cell is one in which the chemical action eats away one of the electrodes, usually the negative electrode. When this happens, the electrode must be replaced or the cell must be discarded.
In the galvanic-type cell, the zinc electrode and the liquid electrolyte are usually replaced when this happens. In the case of the dry cell, it is usually cheaper to buy a new cell.
A secondary cell is one in which the electrodes and the electrolyte are altered by the chemical action that takes place when the cell delivers current.
These cells may be restored to their original condition by
forcing an electric current through them in the direction opposite to that of
discharge. The automobile storage battery is a common example of the secondary
cell.
Electro
Chemical Action
Primary
Cell Chemistry
Secondary Cell
Action
Polarization
Types of
Cells
Other Types of
Cells
Mercury cell
Combining
Cells
Battery
Construction